Beauty equipment safety: Regulations and technological innovations are being strengthened globally
In recent years, the medical aesthetics industry has experienced rapid growth, but with it comes increasing concerns about safety. Many countries and regions have tightened regulations on the industry, standardizing procedures to protect consumer rights. At the same time, technological advancements have made non-invasive and minimally invasive treatments the trend, offering safe, effective, and low-risk beauty solutions.
I. Stricter Global Regulations: Safety as the Core Priority
1. Governments Strengthen Oversight of Medical Aesthetics
-
China: The National Health Commission issued the Medical Aesthetic Service Management Regulations, clarifying facility qualifications and practitioner requirements to crack down on illegal practices.
-
USA (FDA): Strictly approves lasers, radiofrequency devices, and other aesthetic equipment to ensure safety and efficacy.
-
EU (CE ): Requires medical aesthetic devices to comply with medical device directives, ensuring consumer safety.
-
Canada (HC): Regulates medical and aesthetic devices to ensure compliance with safety and performance standards.
-
Brazil (ANVISA): Approves medical and cosmetic equipment, enforcing strict quality and efficacy requirements.
-
Colombia (INVIMA): Oversees medical device registration and safety for consumer protection.
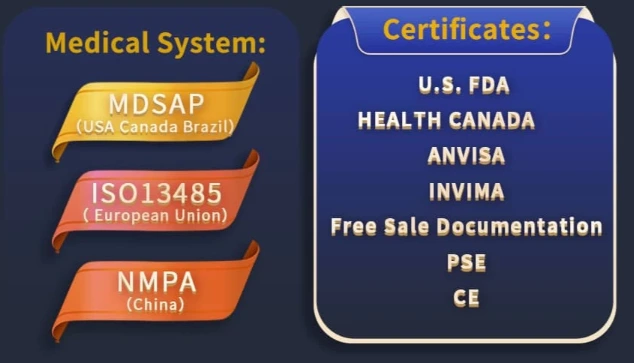
Omni Laser's Medical System and Certifications:
2. Risks of Substandard or Non-Compliant Devices
-
Burns & Scarring: Poorly calibrated laser or RF devices may cause irreversible damage.
-
Infection Risks: Non-sterilized equipment can lead to cross-contamination.
-
Ineffective Results: Low-quality devices fail to deliver promised results (e.g., skin tightening, pigmentation removal) and may worsen skin conditions.
Our Advantages:
✅ Internationally Recognized Certifications: FDA (USA), CE (EU), HC (Canada),ANVISA(Brazil),INVIMA(Colombia) ensuring safety and efficacy.
✅ Strict Quality Control: Complies with medical-grade production standards, minimizing operational risks.
II. Technological Advancements: Low-Risk, Fast-Recovery Aesthetic Solutions
1. Non-Invasive Technologies: No Downtime, Instant Results
-
Radiofrequency Skin Tightening : Stimulates collagen production with zero recovery time; normal makeup and social activities are possible immediately.
-
Cold Plasma Technology: The latest innovation for skin repair, treating acne and fine lines with minimal downtime and no thermal damage.
-
Microneedling: Delivers nutrients through micro-channels, with only 1-3 days of recovery.
-
Thread Lifts: Absorbable threads stimulate collagen for a natural lift, with mild swelling subsiding in 3-5 days.
III. How to Choose Safe Beauty Equipment
-
Verify equipment's Credentials: Check the production standard.
-
Confirm Device Certifications: Prioritize FDA or CE approved equipment; avoid uncertified products.
-
Assess Needs Rationally: Non-invasive/minimally invasive treatments suit maintenance; severe aging concerns may require professional evaluation.

Conclusion
Amid tightening global regulations, the medical aesthetics industry is evolving toward greater safety and efficacy. Choosing compliant technologies and certified devices ensures not only reduced risks but also natural, long-lasting beauty enhancements.
(For details on specific technologies or certifications, feel free to inquire!)
By Elsen